As the essential link between generations, germ cells do one thing and one thing well — produce the gametes (sperm and eggs) required for sexual reproduction. Before generating gametes, most animals make their germ cells once during embryonic development, then squirrel them away for safe keeping until needed for reproduction.
Not so for planarian flatworms — remarkable animals that march to their own biological beat in many extraordinary ways. Planarians fascinate researchers due to their the virtually unlimited capacity to regenerate missing tissue from any part of the body. That regenerative prowess also applies to their germ cells.
Scientists have a limited understanding of how any organisms regenerate germ cells. A new study by Phil Newmark’s Lab at the Morgridge Institute for Research provides molecular insight into how germ cells are regenerated — and may point to new ways to fight their disease-causing relatives, called schistosomes, which sicken hundreds of millions of people annually.

“These animals are so incredibly plastic,” says Newmark, Investigator of the Howard Hughes Medical Institute and Professor of Integrative Biology at the University of Wisconsin–Madison. “They not only have this unusual ability to regenerate their germ cells, but they can regulate them in response to all kinds of physiological cues. Their capacity to trigger germ cell development and gamete production in response to both internal and external cues likely provides a reproductive advantage.”
In work published in the March 15, 2022 issue of the journal Cell Reports, former graduate student Umair Khan and Newmark described intriguing genetic similarities between planarian female germ cells and neoblasts, which are the pluripotent stem cells that drive regeneration. The research suggests that in response to wounding, neoblasts may activate some genetic programs related to female germ cell development.
This study focused on female germ cells because of their unique role in reproductive function. “Female germ cells are perhaps the most interesting of all cell types, because in many cases, they have the ability to create a whole new organism on their own,” Newmark says. “For example, there are animals, including some planarians, that don’t even need genetic contribution from sperm — the eggs themselves contain all the information to make a new animal.”
One interesting finding relates to the planarian’s mode of reproduction; they are hermaphrodites, in which a single animal contains both male and female reproductive systems. “One of the big questions is, how does a germ cell in a hermaphrodite know whether it’s male or female?” Newmark asks. “Before this study, none of our existing genetic markers distinguished between early male and female germ cells.”
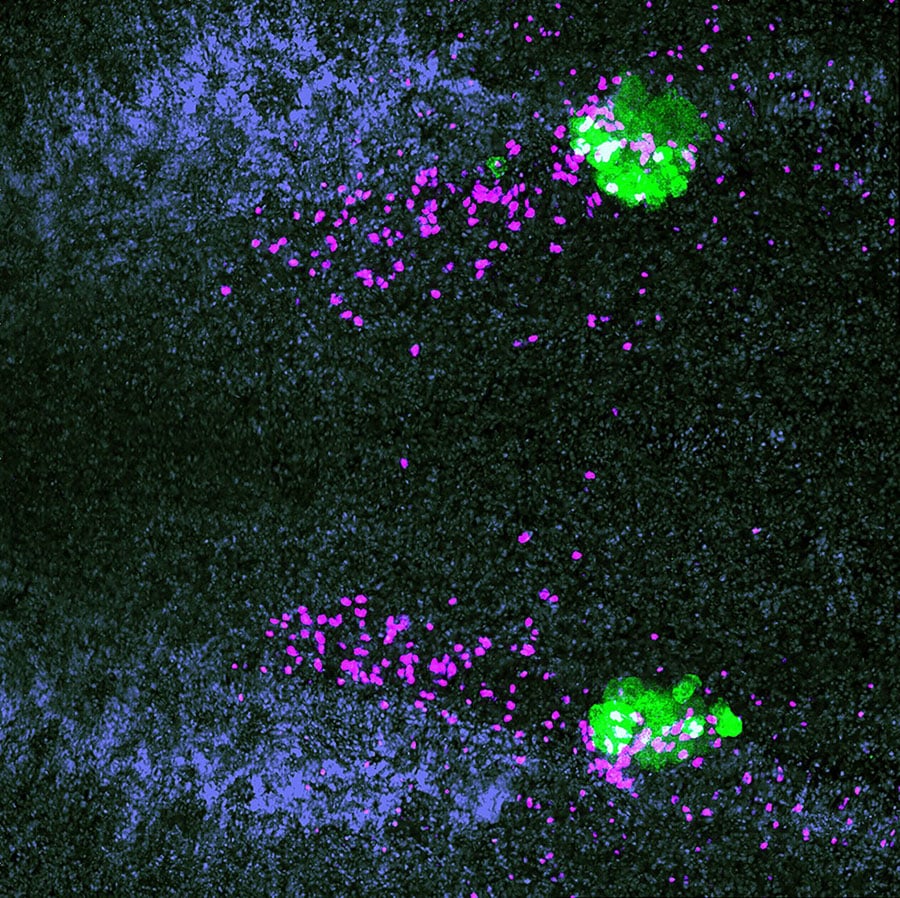
The authors identified a gene expressed specifically in female germ cells, which is expressed early in their development and is required only for female germ cell development. This work provides the first genetic evidence of when a germ cell “knows” to become female rather than male in these hermaphrodites.
The authors also made the surprising discovery that biogenic monoamines play roles in male and female germ cell development. Biogenic monoamines expressed in the brain — such as serotonin and dopamine — regulate functions such as movement, behavior, emotions, temperature and blood pressure in humans. But in the case of planarians, an enzyme that produces monoamines — called AADC — is found in unexpectedly high levels in the planarian ovary.
The researchers inhibited AADC function, which resulted in a failure to regenerate ovaries after amputation; in contrast, it led to testes that abnormally accumulate the earliest stages of male germ cell development, but fail to produce sperm.
“Because inhibition of AADC results in the loss of ovaries and other female accessory reproductive organs, unraveling the role of monoamines in the female flatworm reproductive system may lead to new approaches for preventing parasite transmission,” the study states.
Understanding these reproductive nuances could yield intriguing leads in the quest to fight the debilitating tropical disease schistosomiasis. Schistosomes, for example, only develop female reproductive organs after they are paired with a male schistosome. Schistosomes are a direct descendant of planarians, so the fundamental biology gleaned from planarian work may be directly relevant to fighting this tropical disease. The more researchers learn about flatworm reproduction, the more likely they can find a target to shut down sexual development and thereby terminate parasite transmission.
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health.
