Rebecca Blank and Brad Schwartz
Two decades later, a UW discovery fuels powerful scientific advances
An interest in endangered species led scientist James Thomson to make one of the world’s most profound scientific discoveries.

Brad Schwartz and Rebecca Blank
In 1998, for the first time, Thomson successfully cultured human embryonic stem cells, which are pluripotent – capable of becoming nearly any of the 200 different cells types in the body.
The discovery opened entirely new pathways for exploring basic human biology and treatments for some of humanity’s most intractable diseases.
Thomson, director of regenerative medicine at the Morgridge Institute for Research and a professor of cellular and regenerative biology at UW–Madison, made the discovery not because it was what he was looking for, but because he wanted to help preserve disappearing populations of great apes.
Science is full of serendipity; it just takes the right kind of scientist to see it.
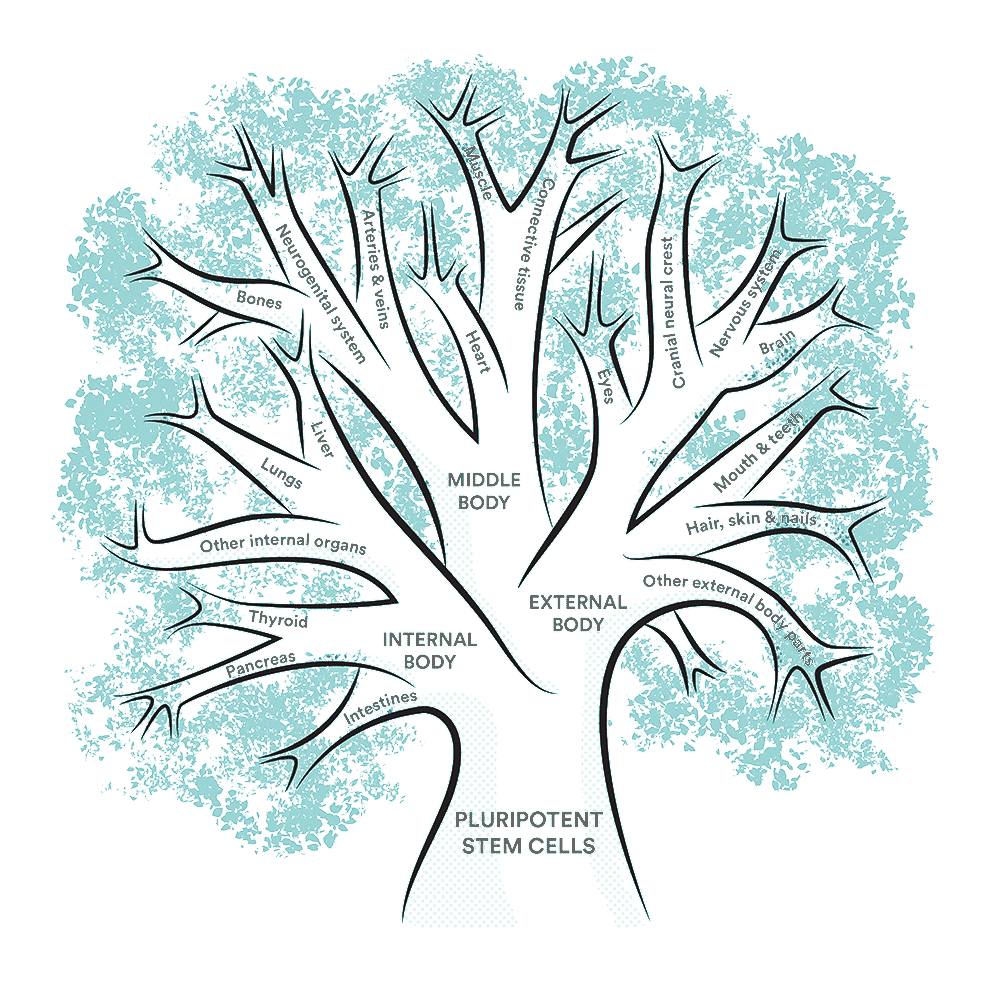
The Stem Cell Tree
- Pluripotent stem cells — the trunk of the tree — are capable of becoming any cell in the human body, making them highly valuable to science and medicine.
- At the big forks of the tree, cells begin to organize into three states: Internal (Endoderm), Middle (Mesoderm) and External (Ectoderm) parts of the body. They still differentiate into many different cell types, but only within those categories.
- Finally, the branches and twigs of the tree represent the cells evolving from parent cells (also called progenitor cells) into the final, highly specific cells needed across the human body. Overall, humans have more than 200 distinct cell types.
Stories
Immortal: An oral history of stem cell discovery
In November 1998, the journal Science published James Thomson’s groundbreaking work on embryonic stem cells. There has been 20 years of progress since the initial discovery spawned a new field of research, and tremendous potential exists for the future. We reached out to the people who lived it, and they shared the experiences in their own words. This is their story.

Out of left field: Thomson looks at discovery and the future of science
James Thomson, the UW–Madison biologist whose stem cell discovery 20 years ago opened fascinating and promising new avenues in science, took time to discuss his thoughts on the breakthrough and what the future holds for the field of regenerative medicine.

Summer science camp expands opportunities for rural schools
The Rural Summer Science Camp has seen more than 500 rural high school students and teachers since its inception in 2007. Campers learn about stem cell science, medical engineering, epigenetics, bioinformatics and more while engaging with and immersing themselves in the work of active researchers at the Morgridge Institute for Research and UW–Madison.

Collaboration leads to ‘dream’ of artery bank
Just as blood banks are essential to medicine, the Thomson Lab hopes to see the advent of artery banks that give surgeons a better, readily available material to replace diseased arteries. The lab is using pluripotent stem cells to grow the cellular building blocks of the artery — endothelial and smooth muscle cells — and coax them into assembling into arteries that can grow and thrive in a majority of patients.
 This is an image of an “organoid” — a tiny, simplified version of an organ in a dish — that includes all the neural tissue components in the brain. These organoids, which self-assemble in a dish, were created by the James Thomson Lab to help test whether drugs or chemicals are toxic to human brain development.
This is an image of an “organoid” — a tiny, simplified version of an organ in a dish — that includes all the neural tissue components in the brain. These organoids, which self-assemble in a dish, were created by the James Thomson Lab to help test whether drugs or chemicals are toxic to human brain development.  Endothelial cells (shown here), engineered from induced pluripotent stem cells, are the building blocks in the James Thomson Lab vascular engineering project. The lab hopes to create functional arteries that can be used in cardiovascular medicine.
Endothelial cells (shown here), engineered from induced pluripotent stem cells, are the building blocks in the James Thomson Lab vascular engineering project. The lab hopes to create functional arteries that can be used in cardiovascular medicine.  These are neural cells assembling in a dish at the James Thomson Lab, creating a model of human brain development. These structures can be used to test drugs for toxicity. The confocal microscopy image shows neurons (green), glial cells (red), and nuclei (blue) within a developing neural construct.
These are neural cells assembling in a dish at the James Thomson Lab, creating a model of human brain development. These structures can be used to test drugs for toxicity. The confocal microscopy image shows neurons (green), glial cells (red), and nuclei (blue) within a developing neural construct.  Cell-cell interaction between endothelial cells (white) and smooth muscle cells (red).
Cell-cell interaction between endothelial cells (white) and smooth muscle cells (red).  A vascular scaffold made of silk. These scaffolds provide a structure around which cells can grow into functional tissue, such as arteries.
A vascular scaffold made of silk. These scaffolds provide a structure around which cells can grow into functional tissue, such as arteries. 
